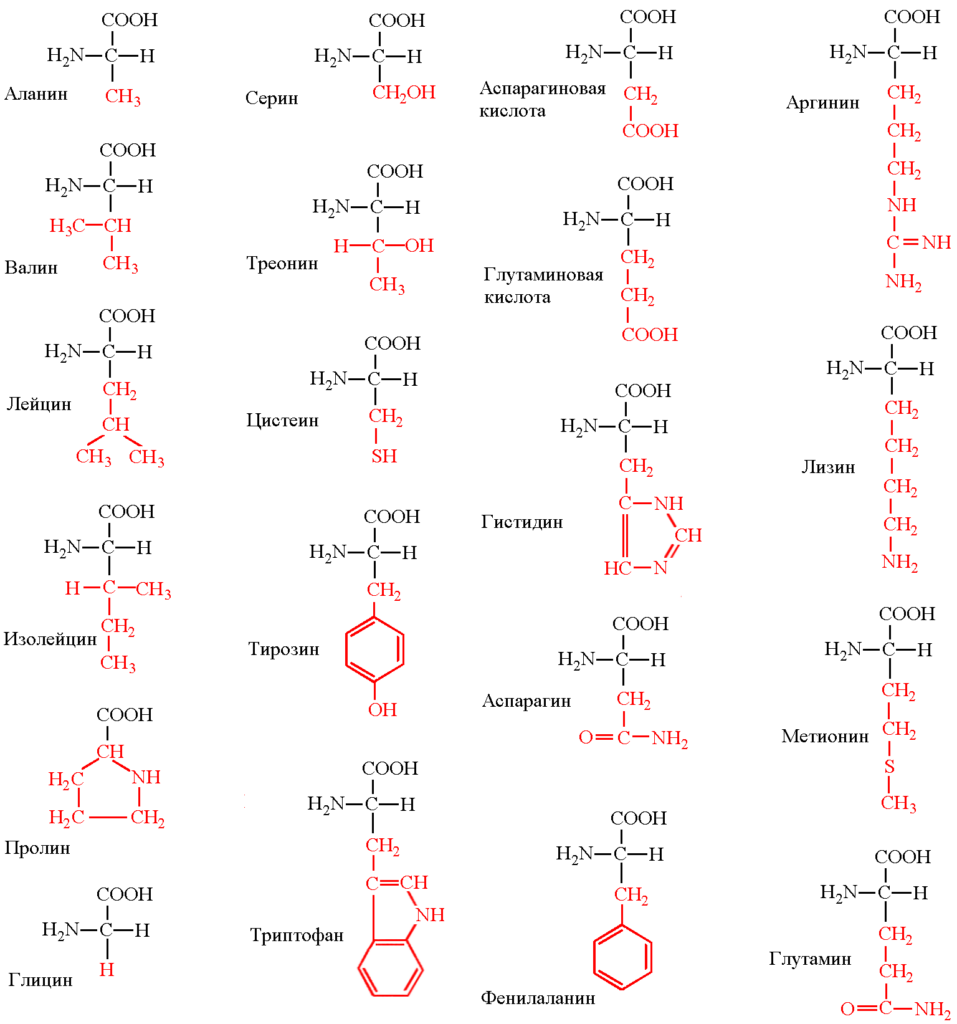
Amino acidsAmino acids are the fundamental building blocks of proteins and play a key role in biological processes. There are a total of 22 standard amino acids used for protein synthesis in living organisms. are organic compounds that are the main building blocks of proteinsProteins are high-molecular organic substances consisting of alpha-amino acids linked in a chain by a peptide bond. In living organisms, the amino acid composition of proteins is determined by the genetic code. During synthesis, 20 standard amino acids are used in most cases. Many combinations of them determine the great diversity of properties of protein molecules. Proteins play a key role in the immune response and can perform transport, storage, catalytic, structural, and receptor functions. Proteins are an important part of the nutrition of animals and humans. The main sources of proteins are meat, poultry, fish, milk, nuts, legumes, and grains.. They play a key role in biological processes such as protein synthesis, cell signaling, and metabolism. Each amino acid consists of certain chemical elements and functional groups. Let’s analyze their structure in detail.
Amino acids are classified according to the properties of their R-groups:
Amino acids are fundamental molecules consisting of an amino group, a carboxyl group, hydrogen, and a unique side chain (R-group). Their diversity and properties determine their role in protein construction and other biological processes. Understanding the structure of amino acids helps to understand their functions and significance for life.